Samsung Bioepis has a Biosimilar Drug Market Report for the Second Quarter of 2024. Some key highlights:
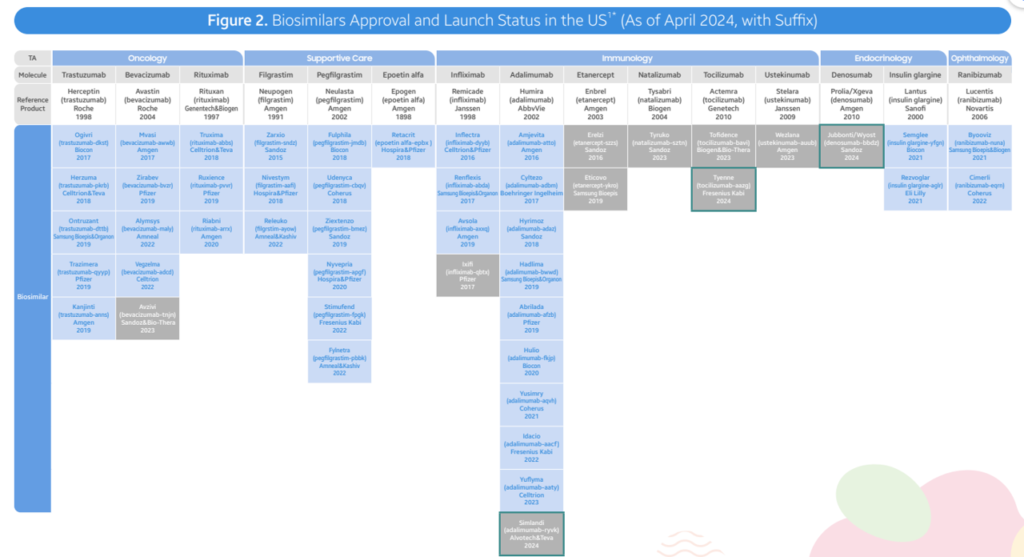
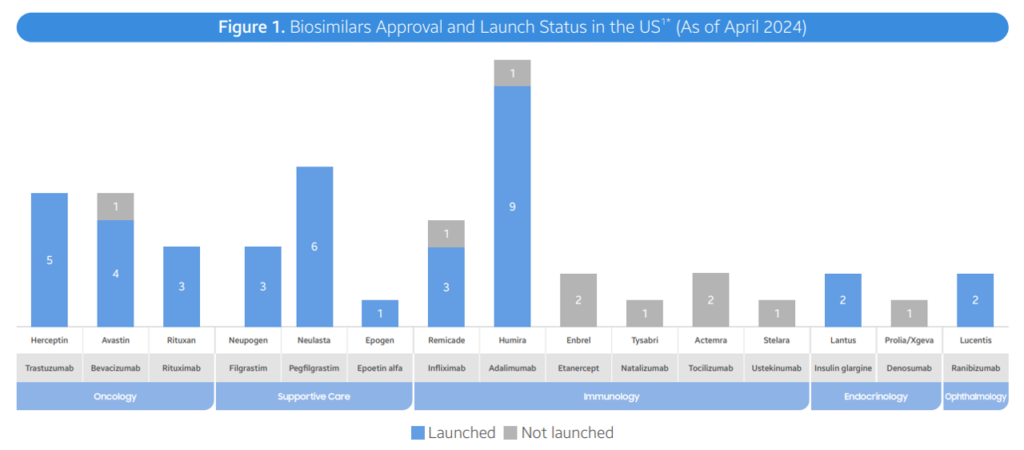
- As of April 2024, the FDA has approved 48 biosimilars of 15 unique biomolecules. Of these 48 approvals, 38 biosimilars are already on the US market. In the second quarter of 2024, 3 new biosimilars were approved in the United States (Simlandi for Humira (adalimumab); Jubbonti/Wyost for Prolia/Xgeva (denosumab); Tyenne for Actemra (adalimumab); Zizumab))
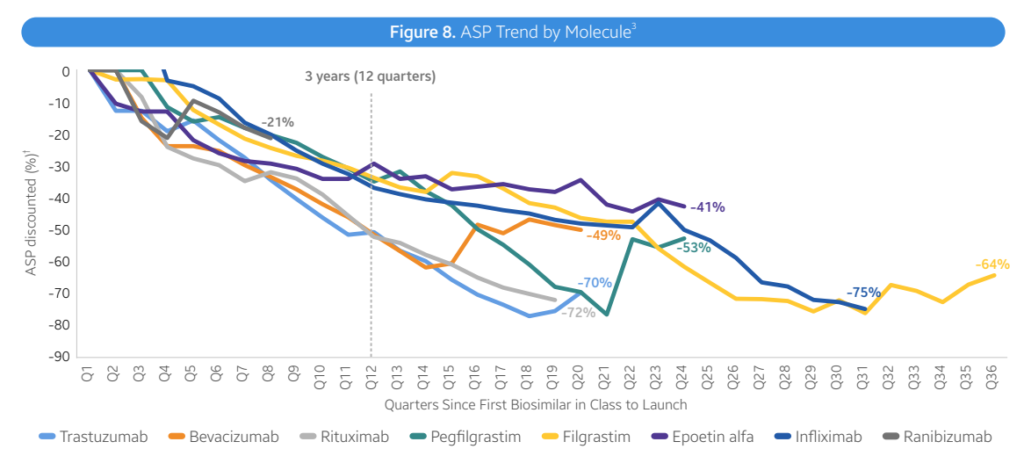
- Moderate discount. Overall, biosimilars are driving down prices, mostly through rebates rather than sticker prices. However, prices have fallen by 41% in 3 years, with wide variations across treatment areas.
- in oncology, the wholesale acquisition cost (WAC) price of biosimilar drugs is 10%-25% lower than that of reference products. In supportive care,
- for supportive carethe WAC price of biosimilars of pegfilgrastim and epoetin alfa is 20-60% lower than the reference price, but the average selling price (ASP) of the reference product has dropped to be comparable to the ASP of the biosimilar , to maintain market share.
- Immunology (Infliximab). Infliximab biosimilars were launched with progressively lower WACs, with discounts ranging from -19% to -59%. Biosimilar competition results in ASP prices being 75-90% lower than reference product WACs
- Immunology (Humira). The adalimumab (Humira) biosimilar takes a different approach. The lower WAC price (85% discount) was used for both biosimilars. Most infliximab biosimilars are launching with progressively lower WACs, with discounts ranging from -19% to -59%. Biosimilar competition results in ASP prices being 75-90% lower than reference product WACs
- ophthalmology. The recently launched ranibizumab (Lucentis) biosimilar has reduced reference product ASP costs by 30-40%. The other six biosimilars follow a similar WAC pricing approach to the Humira brand, but with substantial discounts that reduce the price of the reference product by 55%-90%.
- diabetes. Biosimilars to Lantus (insulin glargine) also have a mixed approach, with some products having lower WACs and others having similar WACs but lower prices due to rebates.
- Absorption of biosimilar drugs varies by molecule. Three years after their launch, the usage rates of biosimilars for bevacizumab, trastuzumab, pegfilgralast and rituximab all exceeded 50%. For other drugs, such as insulin glargine, epoetin alfa and infliximab, the market share in the third year was <50%. In the field, Humira brand products still account for 96% of the market share.
- Inflation reduction method. Some provisions are favorable for biosimilars, while others are less favorable.
- Professional version: Lost revenue to manufacturers from Medicare drug price negotiations and inflationary rebates may lead manufacturers to introduce higher list prices and/or lower rebate rates in other therapeutic areas or lines of business (e.g., private insurance). Biosimilars may provide greater cost relief in these future markets.In addition, the Medicare payment limit for biosimilars is increased to ASP + 8%, helping to offset some of the losses that healthcare providers may incur when using less expensive ASP biosimilars
- scam: Medicare drug price negotiations will put pricing pressure on selected drugs and their related competitors. In these markets (i.e., Enbrel, Stelara), the savings that biosimilars offer plans may be reduced, making step-by-step treatment with biosimilars less attractive for plan sponsors to implement.Additionally, the reduction and cap on Part D member cost-sharing requirements, while positively improving overall affordability for Medicare beneficiaries, inadvertently reduces the financial incentives for members to switch from original products to biosimilars
You can read the full deck here here.